This article will examine some early studies on intraperitoneal injections in mice. These early studies were done to provide a basis for future studies. The main focus will be on proper injection of the mice. We will also cover the clinical signs and effects of intraperitoneal injection. These early studies provide important information on the optimal way to inject mice.
Time of day does not affect anaesthetic depth
The current study provides direct evidence that time of day does not affect the depth of anaesthesia. This finding could have implications for the management of anaesthetics and the outcome of patients undergoing laparoscopic colorectal surgery. The researchers would like to acknowledge the staff at the Nanfang Hospital, Southern Medical University, Guangzhou, China, for their contribution to the study.
Anesthesia depth is critical to the success of surgery. Too light anaesthesia can result in the patient becoming aware of the surgical stimulus and too deep anaesthesia can lead to vasomotor depression and other complications. Clinical signs such as heart rate and blood pressure can provide a rough guide for estimating the depth of anaesthesia, or an estimated anaesthetic plasma concentration can be used as a surrogate. Too much or too little anaesthesia can lead to complications and can even affect the quality of the procedure.
In a study by Kertai et al., researchers found no difference in mortality for patients who underwent surgery during the morning or afternoon. The authors used three thresholds to determine the depth of anaesthesia: BIS 45, MAP 75 mmHg and MAC 0.7.
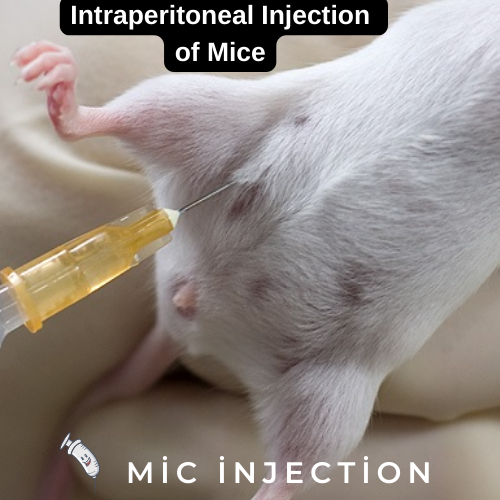
Problems with intraperitoneal injection
One of the major challenges in intraperitoneal injection of mice is the lack of control over the injection site. Steward, Ornellas, and Beernink found that approximately 14% of their intraperitoneal injections were wrong. They considered this error inherent in the technique and not fixable, but they tried to figure out what caused the error. They also found that different sexes and strains of mice grasp the hindlimb differently.
In order to perform intraperitoneal injection, mice must be restrained by the neck region to prevent movement of the mouse. This prevents accidental damage to internal organs during needle insertion. In addition, restraining the mouse by the neck region will shift the abdominal organs toward the diaphragm, avoiding accidental damage.
Clinical signs
Intraperitoneal injections are a way to administer drugs and therapeutics into the peritoneal cavity. They are one of the few methods for administering drugs by injection into a living animal. This technique is used for a variety of research, from drug administration for ovarian cancer to studies on pain in the abdomen after hysterectomy. Understanding intraperitoneal injections will help advance drug delivery methods and open new avenues for research.
Injection of irritants into the peritoneal cavity elicits hallmarks of inflammation, including pain and leukocyte infiltration. The injection also induces the production of inflammatory mediators such as IL-1b and IL-6. The cytokine TNF-a is also induced, which is a marker of immune stimulation. Normally, the peritoneum is protected by the innate immune system. However, injections of irritants can trigger an influx of polymorphonuclear neutrophils.
Intraperitoneal injection of mice is a commonly used method for administering pharmacological agents. However, it raises issues of animal welfare and must be reviewed by IACUCs before the procedure is performed. There are no published studies examining the effects of repeated intraperitoneal injections on animal welfare. One study found that mice did not show adverse effects after receiving 30 i.p. saline injections a day.
Effects on organs in the peritoneal cavity
Intraperitoneal injection of cultured mesothelial cells has been shown to decrease adhesions in mice. Specifically, mesothelial cells decreased the number of adhesions in the CO2 pneumoperitoneum-enhanced adhesion formation model in mice. The cells were isolated from Balb/c mice and cultured for three weeks. The cells were then injected intraperitoneally in a dose-response study using 400,000 cells.
The peritoneum contains a complex lymphatic and vascular network. The majority of these vessels are blood capillaries, while only a few arterioles are present. Microvessel density varies greatly along the peritoneal cavity, with the highest concentration of microvessels in the diaphragm. The diaphragm and mesentery contain 71% of peritoneal microvessels, while the mediastinal peritoneum and parietal peritoneum have less than one-half the number of microvessels in the rabbit diaphragm.
Acute peritoneitis in mice can be induced using zymosan particles, which are insoluble polysaccharides composed of a-mannan and b-glucan components. The presence of these particles induces a synergistic response of leukocytes, as well as cytokines and chemokines. These mediators trigger leukocyte emigration to the inflammatory site.